
New research from the Joint Center for Energy Storage Research (JCESR) puts forth an interesting and probably surprising way to improve battery and fuel cell chemistry: just add water.
Not exactly something that jives with the common knowledge. But JCESR’s research has shown that tiny amounts of water can dramatically improve reaction rates. The water does that by serving as a catalyst for the lithium superoxide (LiO2) during the discharge.
“In our experiment, we can take almost every molecule of water out of the system, and then add it back in molecule-by-molecule to watch how the energy conversion and storage behavior are impacted,” said Nenad Markovic, Argonne Distinguished Fellow in the JCESR Electrochemical Discovery Laboratory.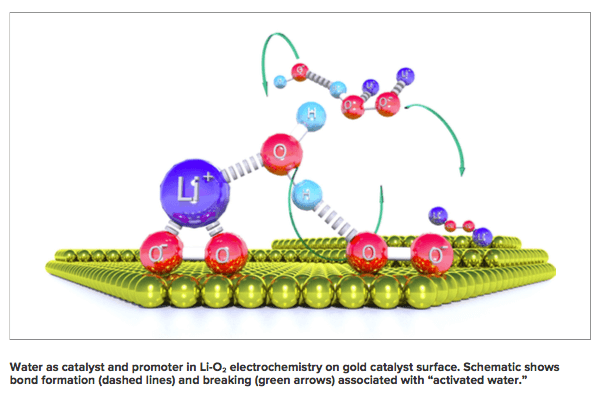 As the water interacts with the lithium superoxide it creates hydrogen peroxide which then creates lithium peroxide (Li2O2). The leftover supplies recombines into water to have the catalyst to use once more.
As the water interacts with the lithium superoxide it creates hydrogen peroxide which then creates lithium peroxide (Li2O2). The leftover supplies recombines into water to have the catalyst to use once more.
In the future, researchers will work on separating the lithium and oxygen from the peroxide and hopefully apply water as a catalyst for other battery chemistries.
Evergreen Climate Innovations is proud to partner with JCESR and provide interactive support for the future of battery technology in our growing clean energy ecosystem. Advanced battery technology offers a dynamic option as an innovative, multi-scale solution that promotes the expansion of clean energy across industries. As a JCESR partner organization, Evergreen Climate Innovations leverages its position in the ecosystem to engage strategic stakeholders and seed pathways for commercialization of breakthrough battery innovations.